Is entropy of universe constant? ///Second law of thermodynamics.
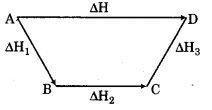
Class 11 Chemistry Is entropy of universe constant? Answer:- No, the entropy of the universe always increases in the course of every spontaneous change. It is the second law of thermodynamics. Define combined form of first and second law of thermodynamics. Answer: - In thermodynamics, the combined law of thermodynamics, also called the Gibb’s fundamental equation, is a mathematical summation of the first law of thermodynamics and the second law of thermodynamics summed into a single concise mathematical statement as dU- TdS + PdV ≤ 0 Where dU is a variation in internal energy. T is temperature, dS is variation in entropy P is pressure dV is variation in volume of a simple working body in which there is neither flows of particles out of the body nor external forces, other than gravity, acting on the body. In theoretical structure, in addition to the obvious inclusion of the first two laws, the combined law incorporates the implications of the zeroth law, via temperat...

