What is Osmotic Pressure
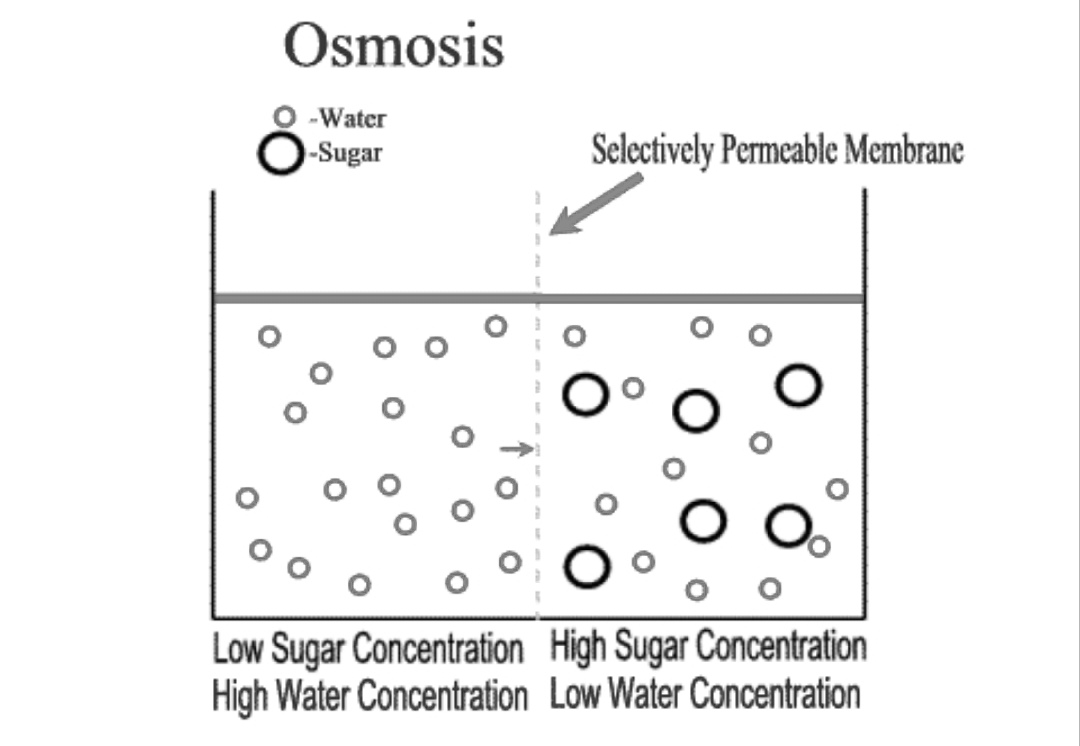
Osmotic pressure is the pressure that we need to apply to stop the flow of solvent molecules from a dilute solution to a concentrated solution through a semi-permeable membrane. Now, the above statement has many terms that need a thorough explanation. We will look at them one by one.

Let us consider the semi-permeable membrane. It is a membrane that selectively allows the movement of particles through it. At some point or the other, we all have seen shrivelling of raw mango when it is pickled in salt water. Blood cells collapse when exposed to saline water.

Q1. Osmosis a reason for cholera?
Ans: Osmosis allows for terrible things to happen, as well. Cholera would not be possible without osmosis. The choleric bacteria populate in our intestines and begin to reverse the intestinal cells’ ionic orientation. In other words, it changes the way ions and, subsequently, water transport in our intestines. It means that cholera performs a perfect coup.

First of all, when our Ions’ orientations are switched, the intestinal cells are no longer able to absorb water into the body. Now osmosis happens in the other direction and water moves from our intestinal cells into our intestines. This is what causes cholera’s infamously deadly watery diarrhoea. Second, this compounds the rate at which you get dehydrated. Not only can you not absorb water, you are literally being drained dry. This is why cholera can kill you so quickly because it does not rely on how much water you consume.



Comments
Post a Comment
Please do not enter any spam link in comment box.